Key control points for in-ovo vaccination
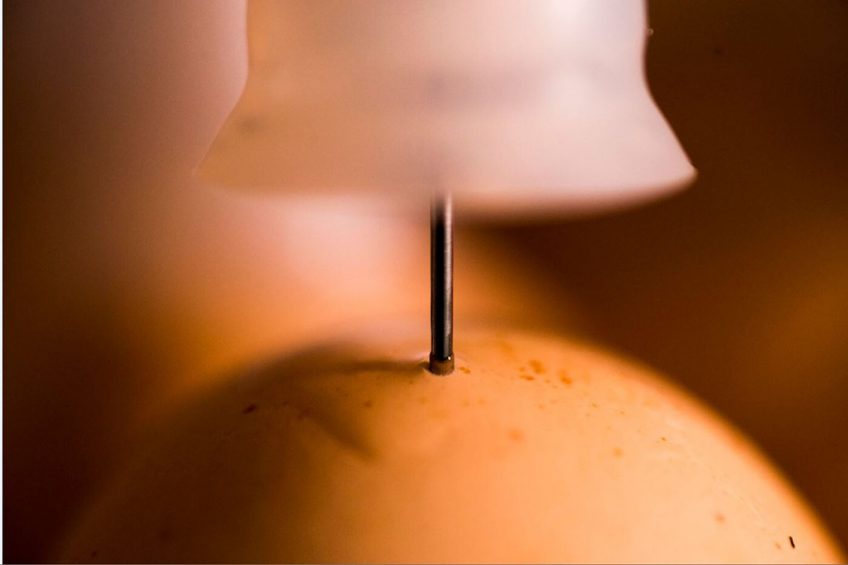
The in-ovo vaccination process is much more than the equipment used for the injection. Indeed, with the growth of this practice worldwide and its potential to ensure massive immunisation, we should pay attention to the way it is done. This is why, defining a good monitoring programme to control the critical factors affecting the process of vaccination is essential for success.
Since the past 30 years, in-ovo vaccination became the preferred method of vaccination in several countries like the USA, Brazil, Spain and Japan. Progressively, this tendency has been adopted by big integrators in many other countries around the world like in Turkey, the Middle-East, and Latin-America. The benefits of this technology were quite obvious, especially in regions traditionally vaccinating against Marek’s disease, and for hatcheries with medium to large capacities and with significant labour costs. But, during the last 4 years, the global poultry industry has really started moving towards this method of vaccination. Currently, the technology is no longer restricted to several countries or big integrators, actually its geo-expansion is a reality.
There are some reasons to explain this. The first is the development of new technology vaccines against the major poultry diseases multi-valent at one-dose for life, which is pulling the in-ovo market to develop in non-traditional in-ovo areas, like north and central Europe, Africa and Asia. Now, by maximising the number of vaccines that can be applied at the same time, the required investment to implement in-ovo becomes affordable and, in fact, is paid back in a short period of time. The second factor is the development of a new generation of in-ovo machines designed to selectively inject and transfer only embryos which are alive while contaminated eggs remain untouched. Indeed, the latest technology allowing the optimum sequence of events during in-ovo vaccination maximises the safety of the injection process by minimising the risk of contamination.
The importance of a monitoring programme
The in-ovo vaccination process is much more than the equipment used. Indeed, with the growth of this practice worldwide and its potential to ensure massive immunisation, we should pay more attention to the way it is done in the hatcheries. This is why, defining a good monitoring programme to control the critical factors affecting the process of vaccination is essential for success.
Since in-ovo vaccination is implemented, the special care of the process should be part of our routine activity in the hatcheries, and it starts at the egg reception and goes up to the hatchability and chick quality assessment. Additionally, the vaccine administration control, process sanitation, microbiological environmental assessments are also a must-have for success. Any deviation will have consequently implications on hatchery performance or field protection.
What to assess and when?
It is all about planification, execution of different testings and monitoring of key performance indicators. Here below, we will explain the key control points and their importance accordingly.

Click here to enlarge this image
1. Vaccine preparation monitoring
To monitor the respect of the vaccine preparation protocols supplied by the vaccine manufacturer are essential to minimize any eventual loss of vaccine quality and vaccine titers. From vaccine preparation room to vaccination equipment, we need to verify:
- The storage conditions
- The operator technique
- The application of aseptic routines
- The good traceability practices
Additionally, the daily dosage control is a key indicator of the equipment’s accuracy level of vaccine dosing.
2. Embryo age diagnosis
There are many different compartments and areas inside the egg (see image below), and vaccines will behave differently depending on which of those areas it is delivered into. The embryo age diagnostic testing is essential to monitor the embryo development of vaccinated flocks at the moment of the vaccination due to its influences in the proportion of these compartments and consequently in the vaccine location site and vaccine efficacy. To maximise target locations (amniotic sac or intra-embryo) the optimum embryonic development (ED) for in-ovo vaccination is 18.5-19 days (Mean >18.5).

Click here to enlarge this image
3. Site of injection assessment
The aim of the site of injection assessment is to validate the location of the injection in the vaccinated egg. Traditionally, standard locations for correct in-ovo are intra-embryo deposition and amniotic fluid location. By nature, the more advanced is the embryo development level, the lower is the volume of liquid at the amniotic sac and higher the probability of locating the vaccine intra-embryo. Both of them are targets. However, according to embryo development this can change, making the vaccine being delivered more or less deep inside the egg that might impact vaccination efficiency or hatchery performance.
4. Dosing accuracy
Uniform and precise 0,05ml vaccine volume needs to be delivered into each embryonated egg. To ensure the full dose is delivered in the target locations, absence of block or bent needles need to be monitored, as well as the proper sliding of injectors and synchronisation between injection and vaccine delivery.
5. Process sanitation & hatchery environment
In-ovo vaccination is sensitive to contamination levels and could influence the transfer and hatch due to a significant increase of the risk of an eventual embryo contamination. Therefore, the incubation process and in-ovo vaccination needs to be conducted maximising the hygiene conditions and following specific procedures to keep contamination levels under control. Hygiene requirements monitoring for in ovo-vaccination are based in the control of the following 3 main pillars:
- Biosecurity, hatchery cleaning & disinfection and environmental control
- Hatching eggs quality and disinfection
- Sanitation during the in-ovo process itself is influenced by the water and sanitiser quality, sanitary status of the flocks, waste management, rotten eggs handling among other factors.

6. In- ovo injection technique
Eggs need to be perforated on the top centre, with no vaccine delivered outside of the egg or egg breakage. Meaning that the following features need to be minimised and therefore monitored because they will affect to the vaccination efficacy or embryo integrity:
- Lateral injections that lead to a risk on embryo integrity as vital organs can be perforated
- Egg breakage, if eggshell is too fragile, there are micro-cracks or hatch baskets are not handled carefully, egg breakage might increase.
- Upside-down eggs that need to be minimised at the time eggs are placed on trays because they will not hatch or will become into cull chicks

The in-ovo C.H.I.C.K Program
Ceva hatchery vaccination services, well known as the C.H.I.C.K Program, aims at ensuring all birds are well vaccinated thanks to dedicated people, equipment and processes that are independently Quality Recognized by the certification and control company, Bureau Veritas Group.
By the regular visits of a Ceva local hatchery specialist, the in-ovo C.H.I.C.K Program is implemented to make sure that the in-ovo technology installed contributes to improving the hatchery performance. Our standardised protocols for every testing performed by qualified personnel are basic tools to the monitoring of the different practices mentioned above including the maximisation of hatchery performance thanks to hatchability and chick quality assessments. According to the hatchery needs, different testing protocols are part of the routine programme of services and its results are made available to producers for data-driven decisions.
Author: Paola Cruz and Miren Arbe, Corporate Vaccination Services and Equipment, Poultry Franchise, Ceva Santé Animale France
Join 31,000+ subscribers
Subscribe to our newsletter to stay updated about all the need-to-know content in the poultry sector, three times a week. Beheer
Beheer





 WP Admin
WP Admin  Bewerk bericht
Bewerk bericht