Using inactivated vaccines to control low pathogenic AI
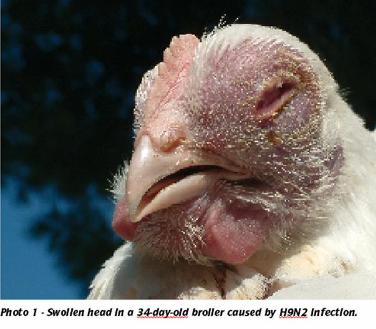
Good quality H9N2 inactivated vaccines, when applied widely within a country or region, may significantly reduce the number of outbreaks and limit economic losses to the poultry industry caused by avian influenza.
By Dr Beny Perelman, Abic Veterinary Company, Israel
In the last decade the threat of a world pandemia caused by the H5N1 influenza virus has become a global issue. Enormous economic losses have resulted from H5 and the H7 influenza viruses in different areas around the world, with hundreds of millions of birds killed by the disease. This, together with the threat of mutation and adaptation of the High Pathogenicity Avian Influenza (HPAI) viruses to humans has forced governments to spend much effort and money in research, surveillance and control measures. The fear caused by the HPAI viruses sometimes obscures the importance of the much larger and more common group of Low Pathogenicity Avian Influenza (LPAI) viruses. LPAI viruses include all the serotypes other than H5 and H7 groups that under laboratory conditions are unable to cause disease or mortality after artificial infection of the virus in birds.
The H9N2 serogroup
The H9N2 serogroup of LPAI viruses is a widespread and endemic virus in Far and Middle Eastern countries. H9N2 viruses have caused serious disease and mortality in birds under field conditions, and have been reported to be able to infect and cause a slight disease in humans. H9N2 was reported in humans in China in 1994 and since then it has become endemic in many countries in the Far and Middle East.
The H9N2 virus is very interesting as it shares some genome characteristic with the H5N1 viruses, and because as LPAI it has been found to be able to infect humans. In most of the countries where H9N2 has appeared, the virus has become endemic, causing sometimes severe disease resulting in heavy economic losses to the poultry industry.
H9N2 infection can occur in all species of domestic birds, including chickens, turkeys and even ostriches (Photo’s 1 & 2). The clinical and pathological signs include swollen heads, sinusitis, respiratory problems with accumulation of a cheese-like content in the air sacs and systemic complications due to secondary bacterial infections. In turkeys and heavy breeders, H9N2 may cause up to 10% mortality and drops in egg production of up to 45%.
Controlling H9N2
Because H9N2 is considered a Low Pathogenic virus, eradication techniques are not applied to control the disease, which enables the virus to become endemic. Vaccination with inactivated vaccines has become a common practice to reduce the economic losses caused by H9N2 viruses. However, as all the H9N2 vaccines in use are inactivated, their efficacy seems to be somewhat limited. Inactivated vaccines are known to induce increased levels of specific antibodies, but are considered unable to cause any local immunity or protection in the respiratory tract.
A long-term observation both before and after the introduction of H9N2 vaccines has demonstrated that the use of good quality H9N2 inactivated vaccines – combined with widespread use and correct vaccination programmes – have significantly contributed to the reduction of the number of outbreaks observed (Figure 1). From a practical point of view, it is well accepted that serologic response is dependant on the species of birds vaccinated. Light breeds respond much better to vaccination, thus producing good levels of antibodies (5-7 HI Titers) even after a single application, while heavy breeders have a weaker response requiring at least 2-3 applications of the vaccine in order to achieve protective titers of antibodies.
A common vaccination programme for H9N2 includes a first vaccination at 12-15 days, a second vaccination at 6-10 weeks of age, and a third vaccination at 18 weeks of age. Breeding flocks are vaccinated three times and may reach antibody titers from 5-7 as shown by the Hemmaglutination Inhibition (HI) test. Turkeys seem to have the weakest response to these vaccines, requiring a minimum of three vaccinations to obtain titers of 3-6 that provide partial protection after field challenge. The health status of the flock, as well as the quality of the application, is critical in order to obtain protective levels of antibodies.
Developing a challenge model
The development of a model for the challenge of chickens with H9N2 virus under laboratory conditions has shown that H9N2 inactivated vaccines are able to induce good levels of systemic antibodies easily measured by the HI test. The new challenge protocol has demonstrated that high levels of antibodies induced after vaccination with inactivated H9N2 vaccines can increase the resistance of vaccinated birds to an artificial infection with a high dose of H9N2 field virus.
A clear correlation was found between the number of times the birds received the inactivated vaccine and the titers of specific antibodies to H9N2 (Figures 2 & 3). A clear opposite correlation was also found between the levels of specific antibodies and the ability of the virus to reproduce in the trachea of infected birds (Figures 3 & 4). Chickens with antibody levels of seven and more by the HI test, were found to be refractory to H9N2 infection directly applied to the trachea of the birds, suggesting that IgG systemic antibodies may in some way reach the tracheal epithelium thus protecting the birds against colonisation of the H9N2 virus.
Lower levels of systemic antibodies are able to significantly reduce the ability of the challenge virus to settle and multiply in the tracheal epithelium. This makes the birds more resistant to infection and significantly reduces the multiplication and spreading of the H9N2 virus into the environment.
This new model, developed by Abic Veterinary Company, has enabled a better understanding of the effect
of the vaccine and the vaccination programmes on the correlation between the levels of antibodies and the dynamics of infection and replication of the H9N2 field virus in chickens.
World Poultry Vol. 25 No. 2, 2009
Join 31,000+ subscribers
Subscribe to our newsletter to stay updated about all the need-to-know content in the poultry sector, three times a week. Beheer
Beheer








 WP Admin
WP Admin  Bewerk bericht
Bewerk bericht