Fighting necrotic enteritis – and winning

Necrotic enteritis, which is already one of the most common diseases in broilers, is expected to increase in response to possible future restrictions in the use of ionophores. Understanding the pathogenesis of Clostridium perfringens is key to building a multi-approach strategy to tackle the disease and reduce its damaging impact.
Caused by a rise in Clostridium perfringens (C. perfringens) population, the disease affects birds from 2-6 weeks old, sometimes through an acute clinical attack, resulting in massive mortalities, or in subclinical form, leading to significant performance reduction.
A specific trait of C. perfringens’ metabolism is its lack of amino acid-related genes, which means it is unable to grow when amino acids are limited. However, C. perfringens counters this by employing an arsenal of virulent factors to promote tissue destruction, the release of nutrients and their rapid uptake, degradative enzymes, toxins, and transporter systems.
Different strains of C. perfringens are classified according to 6 toxinotypes from A to G, each subject to the toxins they produce. Also, in the late 2000s, a pore-forming toxin named NetB was identified. Carried by type G C. perfringens, it has a crucial role in the pathogenesis of necrotic enteritis in poultry.
Disease establishment involves several steps. Prior to disease onset, microbiota composition is well balanced between Clostridium species, both commensal Clostridium and C. perfringens. When virulent C. perfringens strains emerge, however, especially NetB positives, bacteriocins are produced, superseding commensal Clostridium strains. This enables C. perfringens to grow rapidly, feeding on the mucus layer. When C. perfringens cells reach sufficient density (107-109 CFU/g of intestinal content) they produce auto-inducing proteins that upregulate the activation of virulent genes. This process is called “quorum sensing”. Once virulent genes are activated, NetB toxin is produced. By this stage, the mucus layer will have been destroyed, allowing NetB to disrupt the membrane of enterocytes. Ions then pour into the cell, killing it, and allowing C. perfringens to feed on its contents.
Promoting commensal Clostridium strains
As displacing commensal Clostridium strains enables C. perfringens to increase growth, these strains need to be boosted to prevent an overgrowth of virulent C. perfringens.
A trial with turkeys, designed to assess the impact of supplementation with the yest postbiotic, Safmannan, recorded an increase of around 50% in Clostridium in faecal samples, alongside higher counts of Clostridium bifermentans.
Reducing virulent C. perfringens strains
Preventing C. perfringens’ growth from reaching a logarithmic phase is crucial to avoid quorum sensing and the activation of virulent genes.
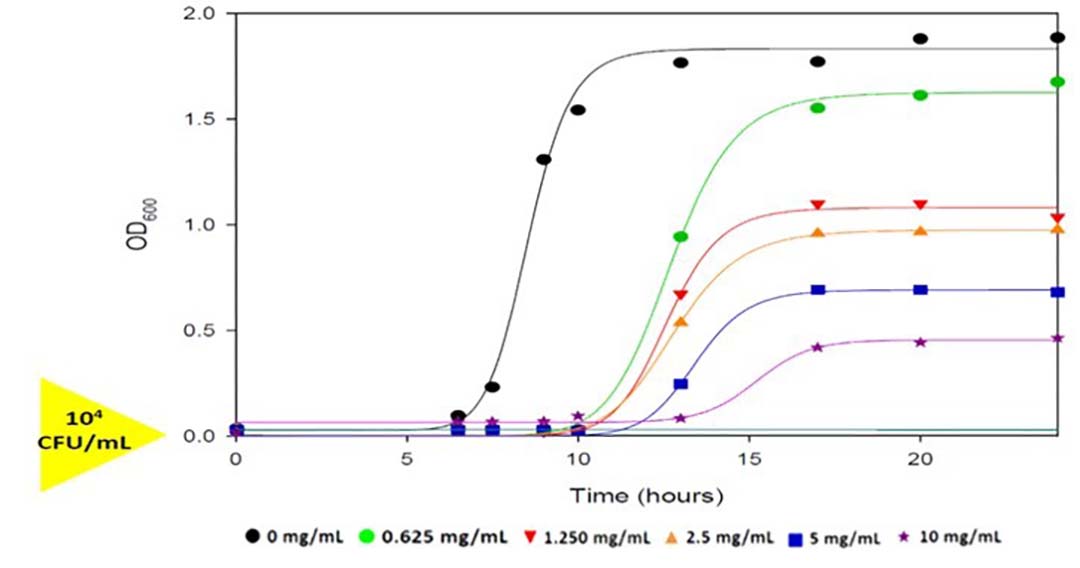
In vitro trials, carried out by Phileo by Lesaffre, have shown the yeast postbiotic has a binding capacity of 75% on C. perfringens. Another study showed that increasing concentrations of the yeast postbiotic in the culture medium extend the lag phase of growth, while also reducing exponential growth, leading to much lower concentrations of bacteria (Figure 1).
Figure 1- Safmannan inhibits Clostridium perfringens growth (Santovito et al., 2019).
Maintaining the protective mucus layer
Given that NetB needs the mucus layer to be destroyed to access the enterocytes, maintaining the mucus layer is key to avoiding tissue necrosis.
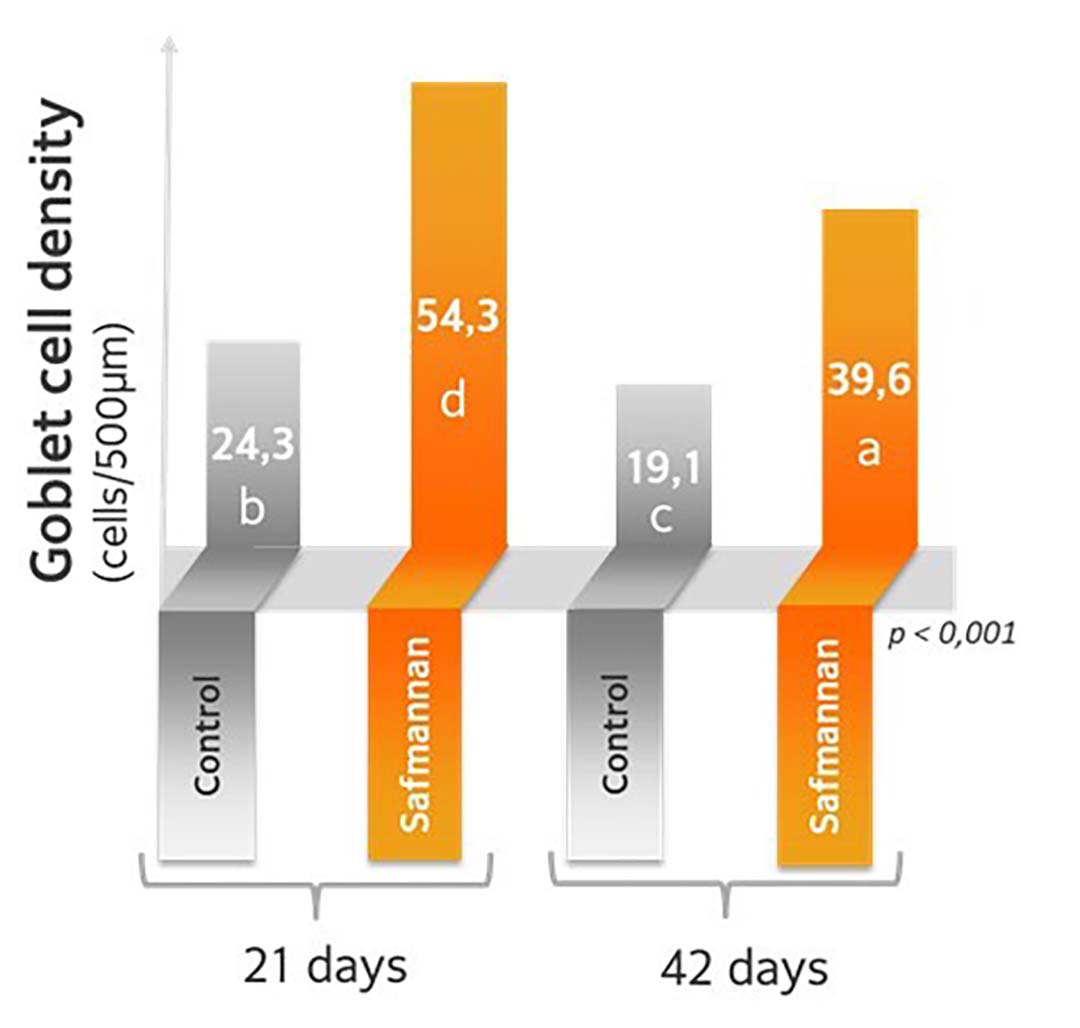
In this context, trials with the yeast postbiotic revealed an increase in goblet cell density of about 50% (Figure 2) and an increase in gene expression for mucin 2.
Figure 2- Safmannan helps protect the gut epithelium (Pascual et al., 2020).
Supporting the immune system
During other studies, the yest postbiotic has been shown to significantly reduce the production of inflammatory proteins while increasing the production of anti-inflammatory proteins. During a trial in China, carried out under an E.coli challenge, the gene expression of NF-κB, TLR4 and interleukin 1-b was significantly reduced by supplementation with the yeast postbiotic. Meanwhile, the gene expression of interleukin 10, known for its anti-inflammatory properties,
was significantly increased. This shows the potential of the yeast postbiotic to prevent further tissue damage.
Other trials have shown an improvement of humoral and cellular immunities, while basophilic hypersensitivity tests and antibody measurements have shown a significant increase in response to the yeast postbiotic supplementation.
Conclusion
Maintaining commensal Clostridium populations, with the yeast postbiotic Safmannan supplementation, helps avoid pathogen overgrowth. When challenged with C. perfringens, birds show improved performance when given the yeast postbiotic supplement in the diet.
The yeast postbiotic has also been shown to have effects on protection of enterocytes from NetB toxin and to preserve enterocyte integrity. In addition to helping reduce C. perfringens population in the gut, the yeast postbiotic promotes the natural defences of birds in their fight against necrotic enteritis.
References are available on request.
Authors: Elen Rondel, Western Europe Poultry manager & Dr Alain Riggi, Global poultry manager Phileo by Lesaffre



