A closer look at the differences between coccidiosis vaccines
Coccidiosis vaccines developed over the years are similar in their objective but vary in many other aspects. This article aims to describe some key elements that differentiate the commercially available coccidiosis vaccines.
The history of the coccidiosis vaccines dates back to 1952, when the first commercial product was launched (William, 2002). The initial work done by Dr Edgar of Auburn University, AL, USA, paved the way for research and development of coccidiosis vaccines to help control this costly disease. The second commercial vaccine, Immucox, was developed and launched in 1985 by Vetech Laboratories, Guelph, Canada. With an innovative delivery system, this product is now registered and sold by Ceva Animal Health in several countries. Since then, several coccidiosis vaccines have been developed and commercialised, most of them made with Eimeria species strains weakened by genetic selection for the European market.
Type of Eimeria strains
Coccidiosis vaccines can be classified into 2 groups; the vaccines containing full-cycles strains (non-precocious) and vaccines formulated with short-cycle strains (precocious).
The full-cycle (FC) vaccines contain Eimeria stains where the live cycle of the parasite has not been modified (Figure 1). The FC vaccines can be divided into 2 groups; vaccine with high oocyst per dose (OPD) and vaccine with low OPD. The FC strains have 2 to 4 schizogony stages (depending on the species), leading to a rapid and robust immune response and production of the optimum amount of oocysts which facilitate the vaccine recycling (booster) in the field. The post-vaccination reaction on FC vaccine depends on the initial number of OPD, the vaccine age (shelf-life), and field management. Due to their optimum reproductive capacity, FC strains can rapidly “seed” the environment and displace field-resistant ones (Snyder et al. 2021).
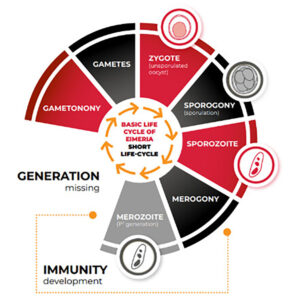
The short-cycle (SC) vaccines contain Eimeria strains (Figure 2) which lost a schizogony stage ( (Shirley, M.W. & Bedrn´õk, P.,1997). Fewer asexual generations result in less gut lesions, reduced parasite fecundity and oocyst output compared to FC strains. The lower oocyst output may slow the onset of immunity under field conditions, impacting flock uniformity and protection against challenge (Mathis et al., 2018). Therefore, SC vaccines demand extra attention regarding field management to promote vaccine cycling and the development of immunity.
Figure 1 – Full-Cycle Eimerias.
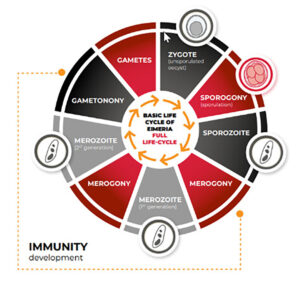
Figure 2 – Short-Cycle Eimeria.
Formulation/ Oocysts per dose (OPD)
Each vaccine has its own formulation, but all must have the most economically important Eimeria species, which are, for broilers: E. acervulina, E. maxima and E. tenella; for breeders/layers: the three species mentioned above plus E. necatrix and E. brunetti.
The OPD differs significantly among the coccidiosis vaccines, ranging from less than 300 to approximately 3,100 oocysts per dose. The shelf-life and the use of SC strains have the highest influence on the OPD.
The OPD is directly proportional to the vaccine shelf-life. The longer the vaccine shelf-life, the higher the OPD will be. For instance, a vaccine with a 10-12-month shelf-life may have up to 8-10 times more OPD when compared to a vaccine with a 6-7-month shelf-life. This is due to the sporozoite viability. The longer the vaccine is stored, the less viable a sporozoite becomes, consequently lessening the potency of the vaccine as it ages. Viability is species-specific; each Eimeria species have its own viability decays pattern. For example, E. maxima depletes its energy more quickly than other species. For this reason, coccidiosis vaccines with long shelf-life (> 9 months) tend to have high OPD to ensure enough sporozoites will have the energy needed to infect the birds at the end of their shelf-life. However, the variation in the vaccine potency as it ages may cause inconsistency in the post-vaccination reaction; the coccidiosis vaccine with high OPD tends to be more reactive when first released than when it is within the last third of its shelf-life. This inconsistency is not observed in the FC vaccine with lower OPD.
The SC Eimeria strains have reduced oocyst output. Therefore, it appears that the high OPD in the FC vaccine is to attempt to compensate for low fecundity and oocyst output. The combination of precociousness and drop in potency due to long shelf-life must also be considered.
Vaccine take
Coccidiosis vaccination is a controlled exposure process. That objective is for all chicks to be exposed to all Eimeria species at the correct dose following vaccination. Chicks that do not receive the vaccine in the hatchery will then be exposed to uncontrolled amounts of vaccine or field oocysts in the environment after the first cycle (Tensa & Jordan, 2018). . Therefore, uniform vaccine uptake is crucial to initiate an immune response to all species in the vaccine.
Live coccidiosis vaccines can be delivered to chicks via topical spray (water or gel droplets), drinking water, in ovo, or on-feed application (Price, 2012). Regardless of the method used, the oocyst must be ingested.
The most common coccidiosis vaccination practice is via water spray or gel droplets. Chapman et al.(2002) and Price et al.(2014) described that a water spray vaccination could result in uneven application. Tensa & Jordan (2018), comparing these 2 methods, reported that ~50% of the coccidiosis vaccine did not reach the chicks when water spray was used; with gel droplets, almost all of the vaccines reached the chicks.
Successfully preventing coccidiosis
Various commercial coccidiosis vaccines are available in the market, and a proper understanding of the specifics of their biology and proper administration is critical for the successful prevention of coccidiosis.
References are available on request.
Join 31,000+ subscribers
Subscribe to our newsletter to stay updated about all the need-to-know content in the poultry sector, three times a week. Beheer
Beheer





 WP Admin
WP Admin  Bewerk bericht
Bewerk bericht