Mycoplasma rFPV-MG vaccine reduces antibiotic usage

Mycoplasma gallisepticum (MG) infection can cause respiratory disease in commercial poultry, particularly in chickens and turkeys. A vector fowl pox virus-based Mycoplasma gallisepticum vaccine has shown positive results in field trials.
The clinical signs of MG can vary depending on the severity of the disease, the age and health of the bird population, but some common signs include sneezing, coughing, nasal discharge, swollen sinuses and/or eyes, reduced feed and water consumption, decreased egg production or quality, poor growth rate and increased mortality.

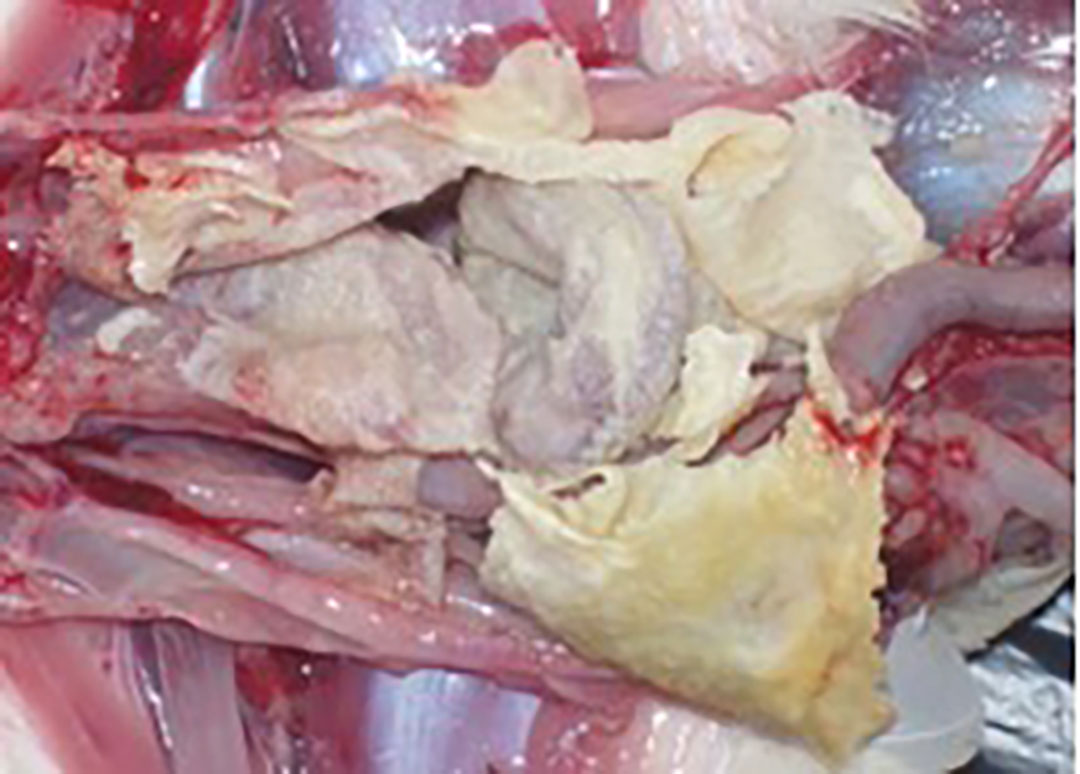
The transmission of MG among birds occurs both horizontally and vertically, widely affecting poultry producing companies. The economic impact of the disease can be significant and is usually associated with decreased productivity, increased mortality, and augmented treatment costs.
Effective management strategies such as vaccination and biosecurity measures can help reduce the impact of MG infection on bird’s health and productivity.
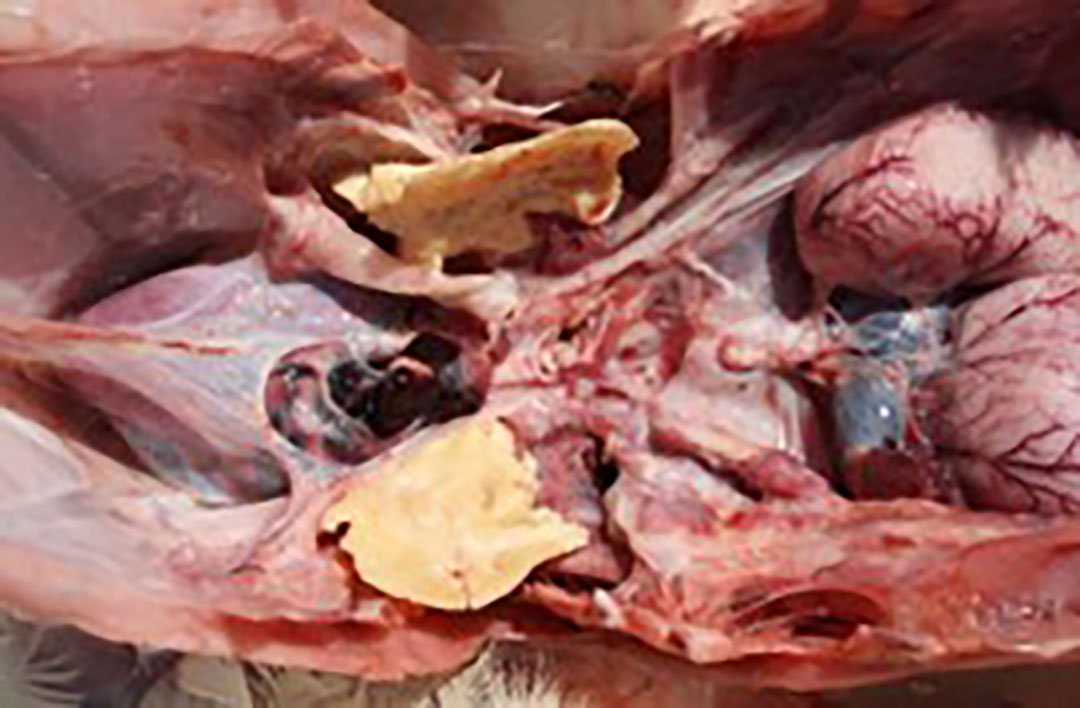
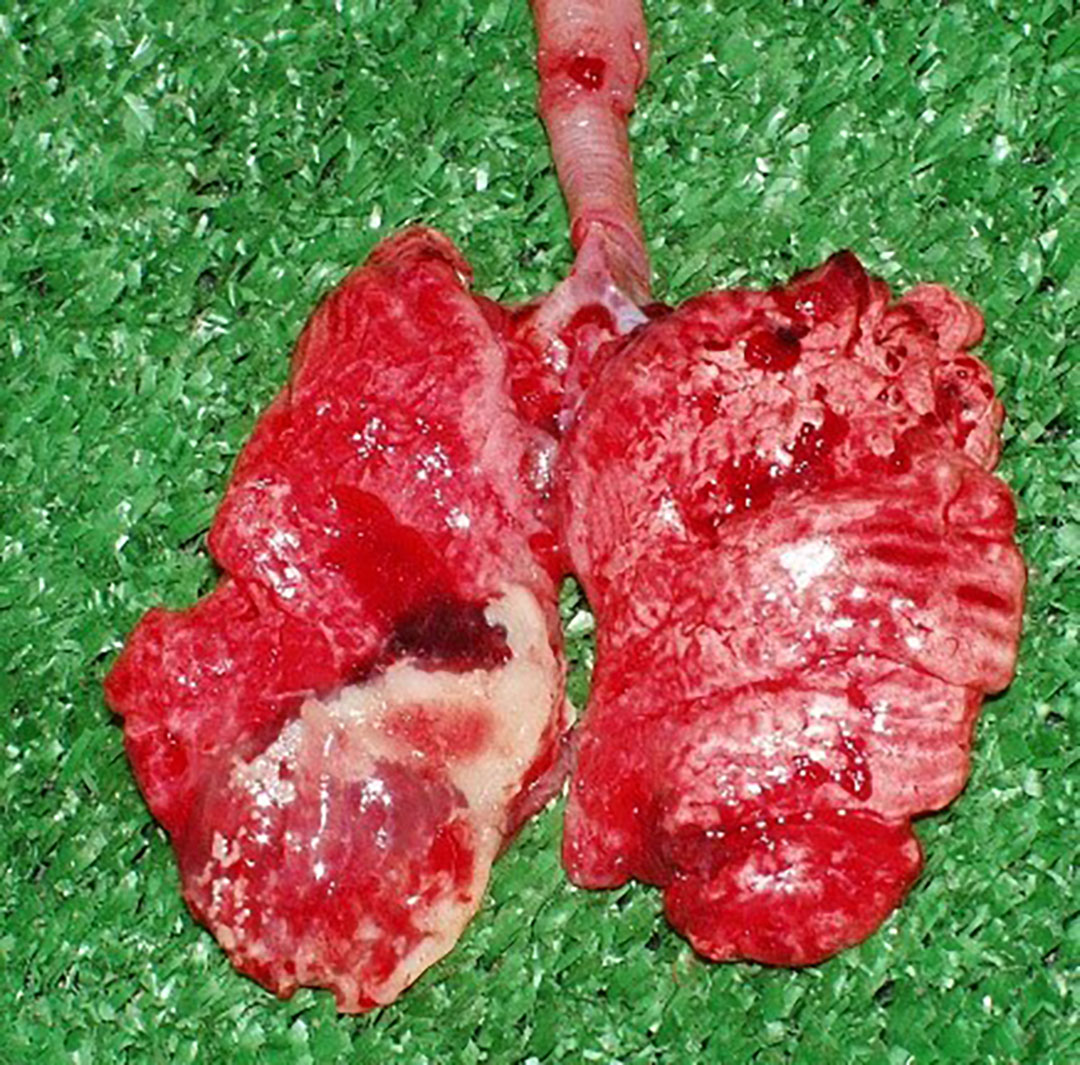
Field study in India
The objective of a field study was to assess 2 different management strategies in high MG pressure areas in India. Two groups with different MG control strategies were compared. The same 11 broiler breeder flocks were monitored with the 2 different control strategies based on the addition or not of a rFPV-MG vaccine.
In the first strategy, 11 flocks were evaluated, identified as F1 to F11. These flocks received several antibiotic treatments throughout their lives and were also vaccinated with inactivated MG vaccines, but no vector MG vaccine was included in the vaccination programme. All these flocks received ‘flushing’ antibiotic treatments at 1 and 3 weeks of age.
Recurrent MG clinical signs, like airsacculitis, were observed during production period in these flocks. Then, Flocks F1 to F3 received antibiotic treatments through water or feed every 4 weeks. Flocks F4 to F7, on top of the antibiotic medication every 4 weeks, were vaccinated with 2 doses of killed MG vaccines. Finally, flocks F8 to F11 received 2 injections of killed MG vaccine and antibiotic medication every 3 weeks.
The second phase of the field study consisted of following-up their immediate replacement flocks, identified as F1a to F11a. All these flocks were vaccinated with a vector fowl pox virus-based Mycoplasma gallisepticum vaccine (rFPV-MG), commercially known as Vectormune FP-MG. Flocks received antibiotic treatment depending on the needs. Flocks 1a to 5a did not receive any dose of MG killed vaccine, whereas flocks 6a to 11a had at least one dose of this type of vaccine in their preventive programme (Table 1).
All flocks were carefully monitored for the rFPV-MG vaccine-take through assessment of granuloma/nodule formation at injection site 5-7 days post-vaccination. Serology using BioChek MG ELISA test was carried out before the vaccination with rFPV-MG and after vaccination with intervals of 10 weeks up to 60 weeks of age. All flocks were observed daily to record any clinical signs attributable to MG and the egg production.
Vaccination quality and vaccine-take
The assessment of the vaccination quality was done by the observation of a granuloma at the site of injection in the wing web at 5-7 days after the application of the rFPV-MG. All flocks had above 96% of vaccine-take and did not show any negative vaccination reaction.
Serology analysis
For interpretation of the MG ELISA results, flocks were categorised into 3 groups based on the use of MG killed vaccines. Flocks F1a to F5a, which did not receive a MG killed vaccine, were included in Group I while flocks F6a to F11a, which were injected with at least 1 dose of MG killed vaccine, were included in Group II.
In Group I, the flock F1a had mean titers below positivity titer up to 60 weeks of age without any MG clinical signs, indicating that there was no MG challenge to the birds. Flocks F2a, F3a, F4a and F5a showed mean titer above the positivity limit (cut-off point) at 40 and 50 weeks of age, indicating MG field infection (Figure 1). However, no clinical signs of MG or drop in egg production that could be associated with it were noticed.
Figure 1 – BioChek MG ELISA monitoring results of flocks vaccinated with rFPV-MG (group 1), rFPV-MG + 1 inactivated MG vaccine (group 2) and rFPV-MG + 2 inactivated MG vaccines (group 3).
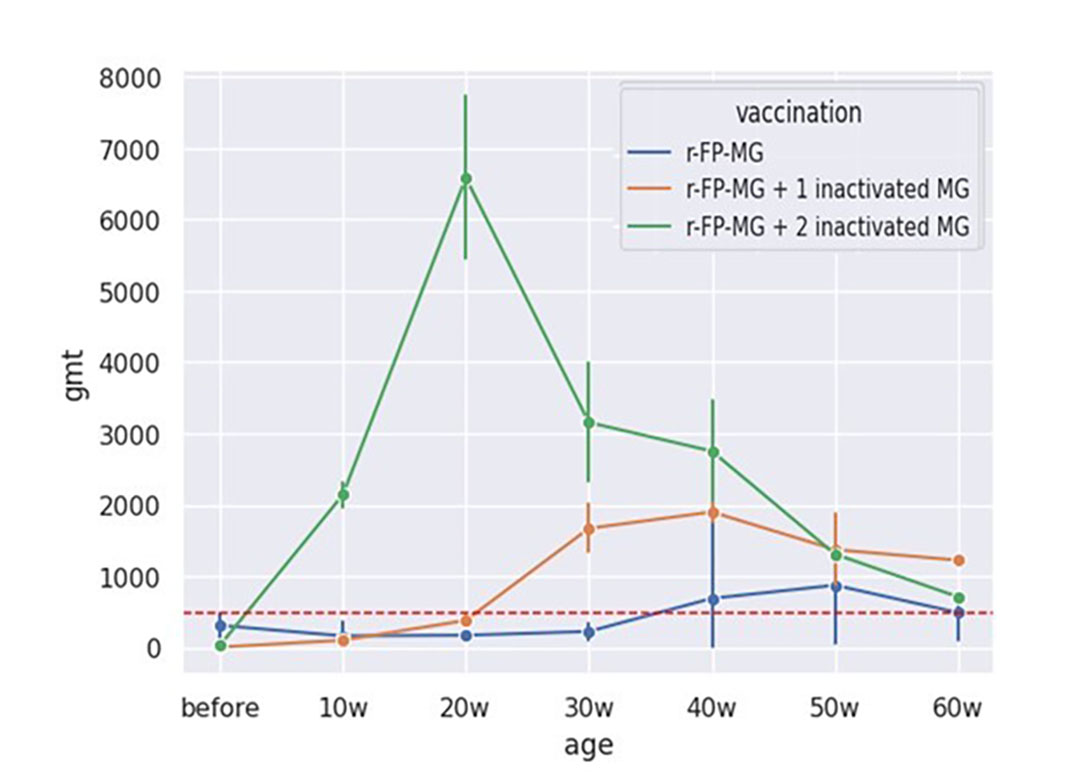
Interestingly, as the rFPV-MG vaccine does not induce any detectable antibody response by commercial MG ELISA kits, this serological test can be used as a DIVA tool in rFPV-MG vaccinated flocks to identify the challenge period or age and this information can be used to strengthen the biosecurity procedures.
In Group 2 and 3, all flocks had positive ELISA titers compatible with the expected antibody response to 1 or 2 doses of inactivated MG vaccines. Moreover, none of them presented clinical signs attributable to MG, indicating the protection of the birds from the vaccines and medication.
Production parameters
All flocks vaccinated with the rFPV-MG vaccine had good productivity results within the expected range from their respective companies. The flock F1a which was vaccinated with rFPV-MG alone had 3 additional hatching eggs compared to the genetic standards. The flock F6a, vaccinated with rFPV-MG vaccine and 1 dose of MG killed plus antibiotic treatments, had 1 additional hatching egg compared with the previous flock in the same campus that was not vaccinated with the vector MG vaccine. Other flocks’ production data were not shared for the study.
Antibiotic reduction with rFPV-MG vaccination
In this field study, the rFPV-MG vaccine reduced the usage of antibiotics in all farms (Table 2).
The introduction of the rFPV-MG vaccine allowed the reduction of antibiotic usage (varying from 4 to 14 antibiotic treatments) in all broiler breeder flocks without compromising their performance. The saving varied from 5 to 35 INR per bird (€55 to €390 per 1,000 birds). On top of the economic benefits for producers, the reduction of antibiotic usage decreases the residues in the eggs and the risks of the development of resistance.
Study conclusions
With the data obtained from monitored broiler breeder flocks, it is possible to conclude the following about the use of Vectormune FP-MG vaccine:
- Vaccination quality can be easily assessed by the vaccine-take (granuloma formation in the wing web). Moreover, no post vaccination reactions were observed.
- Vaccinated flocks did not show clinical signs, even in those flocks that were challenged by MG.
- The vaccine does not induce any detectable antibody response allowing the use of serological tests as DIVA tools.
- Economic benefits were obtained through the reduction of antibiotic treatments compared to control groups. Moreover, it reduces the possibilities of MG antibiotic resistance.
- Vaccinated flocks showed better hatching egg production compared to control groups.
In summary, Vectormune FP-MG proved to be a safe and effective vaccine, providing value and economic benefits in the vaccinated broiler breeder flocks.
Join 31,000+ subscribers
Subscribe to our newsletter to stay updated about all the need-to-know content in the poultry sector, three times a week. Beheer
Beheer





 WP Admin
WP Admin  Bewerk bericht
Bewerk bericht